IIT-M Generates Hydrogen From Seawater
Context:
IIT-Madras physics department researchers have created key elements for a highly efficient, economically viable method of electrolyzing saltwater to produce hydrogen. The findings were released in the ACS Applied Energy Materials journal.
Points to Ponder:
- The traditional alkaline water electrolyzer method uses fresh water for electrolysis, consumes a lot of energy, and necessitates an expensive oxide-polymer separator.
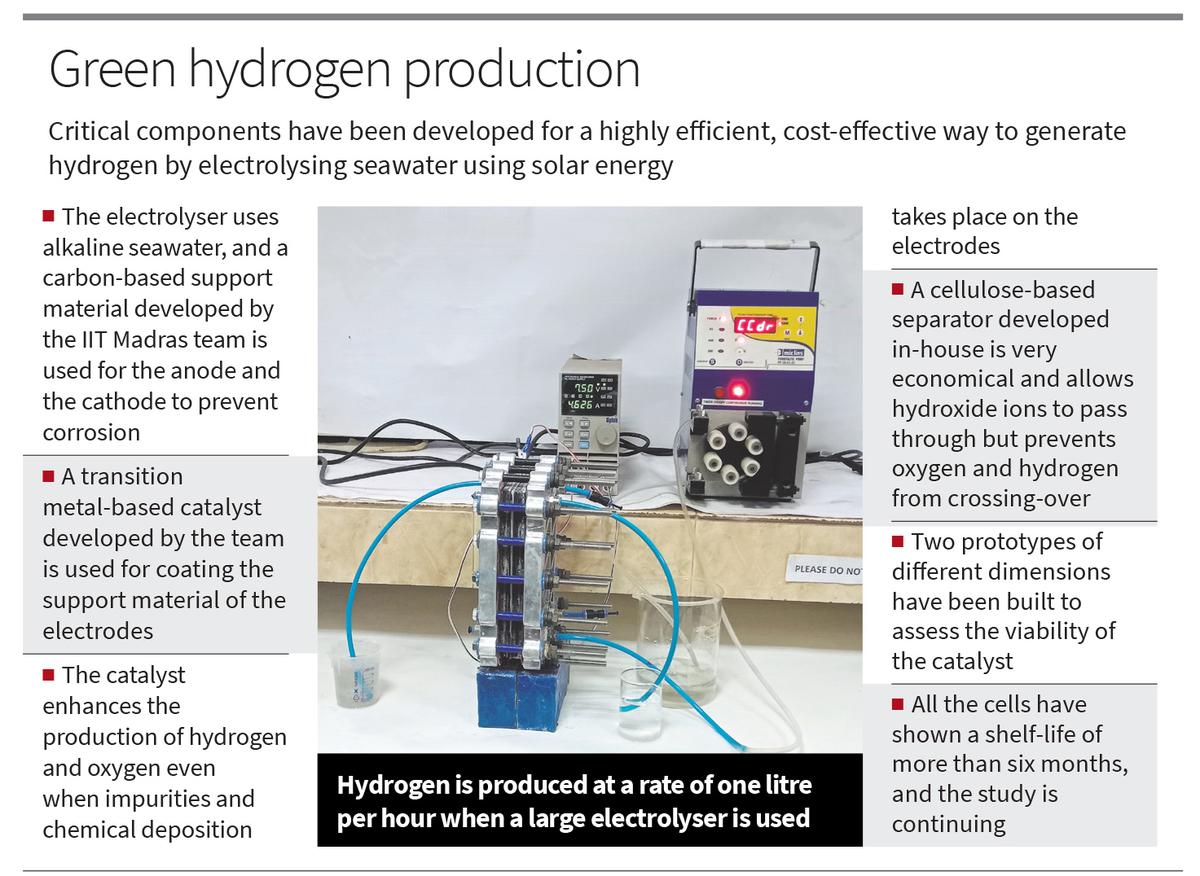
- The IIT-Madras team created an electrolyzer that is more environmentally friendly and utilises less freshwater by using alkaline salt water as the electrolyte rather than pure or fresh water.
- To prevent corrosion, which is a frequent problem when using seawater, they employed a carbon-based support material for the electrodes instead of metals.
- To increase the production of both hydrogen and oxygen even in the presence of impurities and chemical deposition on the electrodes, the researchers designed and created transition metal-based catalysts that can catalyse both the oxygen and hydrogen evolution events.
- When saltwater is used for electrolysis, hypochlorite production at the anode can result in corrosion of the electrode support material and a decrease in the amount of oxygen generated. This problem is addressed by the team’s catalysts, which are more selective towards the oxygen evolution process than hypochlorite production.
- The group created a cellulose-based separator that permits hydroxide ions to move from the cathode to the anode but inhibits hydrogen and oxygen from crossing across. This separator is reasonably priced and does not degrade in seawater.
- Under one sun irradiation at 26 degrees Celsius, the researchers optimised several parameters to obtain a seawater splitting voltage of 1.73 V at 10 mA/sq.cm, or about 12% solar-to-fuel conversion efficiency.
- They showed how to directly use photovoltaic power for saltwater electrolysis, producing 250 ml of hydrogen per hour in a smaller electrolyzer (16 sq cm in size) and about a litre per hour in a bigger electrolyzer (391 sq cm in size) at an applied voltage of 2 V.
- Additionally, the team created a stack of three cells with a production rate of hydrogen of about four litres per hour at an applied voltage of two volts per cell.
- These developments provide a highly effective, affordable, and environmentally friendly way to produce green hydrogen from seawater electrolysis, which could help develop renewable energy sources and lessen dependency on fossil fuels.